Delivering molecules into plants has long been difficult because there are two doors to pass: the cell wall and the plasma membrane. A self‑assembled micellar nanoparticle decorated with aspartic acid (Asp/PDPA‑NP) changes that assumption. Simply spraying leaves or co‑culturing the particles—without special equipment, high pressure, or ultrasound—gets cargo into the plant cytosol within 10 minutes. The particles use the plant’s own amino acid transporters AAP1/LHT1 as the “entry port,” followed by clathrin‑dependent endocytosis. When the plant hormone abscisic acid (ABA) is loaded, it is reliably delivered inside cells, eliciting drought tolerance even at 1 nM, and the approach works in major crops like soybean and maize.
Reference: Amino acid transporters mediate autonomous delivery of nanoparticle vehicles into living plants
- Background: Why Is Delivery into Plants So Hard?
- What Is the Carrier and How Fast Is It? Self‑Assembled Micelles Do the Job
- How Does It Pass? A Two‑Step Strategy: Looser Cell Wall + Endocytosis
- How Far Does It Reach? Cross‑Species and Cross‑Tissue Universality
- How Effective Is It? Performance with ABA
- Summary: Practical Applications Worth Trying
Background: Why Is Delivery into Plants So Hard?
Plant cell walls are tight polysaccharide networks; the effective free‑diffusion size is generally < 5 nm—very small. Beyond that lies the lipid bilayer of the plasma membrane, another barrier. Historically, delivery has leaned on ultra‑small particles or mechanical forces, which limit field spraying and biocompatibility. The present approach is heavy‑metal‑free and force‑free, enabling intracellular delivery with ordinary spraying.
What Is the Carrier and How Fast Is It? Self‑Assembled Micelles Do the Job
The strikingly efficient carrier is a self‑assembled micelle formed in water by an Asp‑PEG‑PDPA block copolymer. With a membrane‑impermeable fluorescent dye (DiO) as cargo, the average size is ~166 nm; with ABA, ~134 nm. While that sounds large relative to the two barriers, delivery still occurs. The drug‑loading efficiency is ~71% on average, and release proceeds gradually over tens of hours. Time‑lapse imaging shows uptake begins in < 10 minutes, with signals spreading into tissue depths over the following hours.
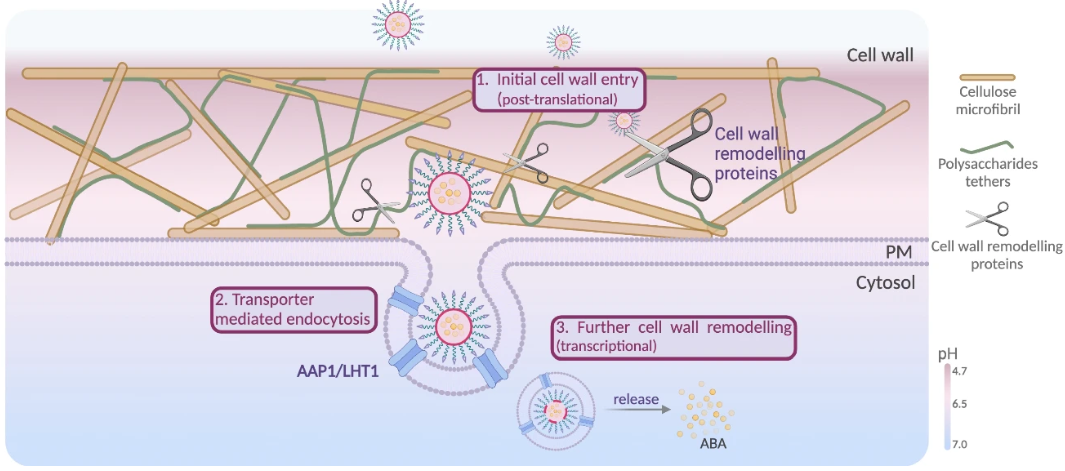
How Does It Pass? A Two‑Step Strategy: Looser Cell Wall + Endocytosis
In practice, how do cargos traverse the wall and membrane? The mechanism is two‑step.
Step 1: Make the Cell Wall Easier to Traverse
Particles tagged with Asp show cytosolic‑side signals more readily than control particles. Leaf transcriptomics further reveal a transient rise in cell‑wall remodeling genes. Together, these findings suggest that shortly after exposure, the cell wall “loosens” a bit, creating paths for passage.
Step 2: Let the Membrane “Engulf” the Particle
The next gate is the membrane. Here, the plant’s amino acid transporters AAP1 and LHT1 act as the entry port. Asp‑decorated particles engage these transporters and are internalized via clathrin‑dependent endocytosis (coated‑vesicle uptake). Experimentally, the endocytosis inhibitor TyrA23 reduces uptake, and in aap1 or lht1 mutants the ABA‑on‑particle response (e.g., RD29A induction and improved drought tolerance) is weakened—evidence supporting this model.
Putting it together: the cell wall first shows signs of “loosening,” creating a route, and then the membrane is opened from the inside via transporter‑mediated endocytosis—a two‑step path that brings nanoparticles into plant cells.
How Far Does It Reach? Cross‑Species and Cross‑Tissue Universality
Beyond Arabidopsis, cytosolic delivery is observed in soybean (dicot) and maize (monocot) leaves and roots. In Tradescantia (ornamental plant), uptake occurs even on the adaxial leaf surface (with few stomata), implying passage through the cuticle without wetting agents. Seeds also show a physiological response (delayed germination), indicating that delivered cargos actually work in situ.
How Effective Is It? Performance with ABA
Efficient trafficking is one thing; how much cargo reaches the cytosol is the key. In leaf compartment assays, ABA on particles shows ~39× higher levels in protoplasts and ~69× higher overall cross‑wall + membrane delivery than free ABA. Phenotypically, 1 nM–1 µM particle‑loaded ABA confers drought tolerance comparable to 1 mM free ABA, with leaf‑temperature increases (stomatal closure) and longer survival also seen in soybean and maize. In short, the required dose drops by orders of magnitude.
Summary: Practical Applications Worth Trying
Spraying Asp‑tagged micellar nanoparticles (Asp/PDPA‑NP) lets plants use their own AAP1/LHT1 transporters to internalize the particles and get into the cytosol within 10 minutes. It works in leaves and roots, in both mono‑ and dicots, and—with ABA—triggers drought tolerance at ultra‑low doses (≥ 1 nM). The novelty is bypassing the two major barriers (wall → membrane) without ultra‑small particles or external forces. Next steps for real‑world use include field‑scale optimization (spray conditions, pH, wetting), long‑term safety and environmental fate, cost, and regulatory considerations.
Where This Could Be Used
- Micro‑dose drought management (demonstrated): single sprays of ABA‑loaded particles ahead of heat or low‑rain periods, reducing transpiration and preserving water with far lower amounts.
- Fine‑tuning germination timing (demonstrated): apply to seeds to induce germination delay, e.g., mitigating pre‑harvest sprouting risks.
- Live visualization of physiology (demonstrated): even membrane‑impermeable dyes can be carried into the cytosol, enabling in‑leaf tracking of movement and diffusion.
- Localized PGR delivery (to be validated): carry other hormones (auxin, cytokinins) for organ‑specific micro‑dosing to fine‑tune traits.
- Lower‑dose biostimulants (to be validated): deliver amino acids or small molecules to target tissues to optimize dose and frequency.
- Toward nucleic acids and proteins (to be validated; challenging): with design tweaks, siRNA/peptides might be deliverable—potentially supporting next‑gen genome‑editing tools.
New technologies and ideas like this just might change our everyday lives.


コメント